
Top Performing Drug – Xtandi (December Edition)
Shots:
-
In continuation of our previous series on the Top-Performing Drug of the month, based on 2021 revenue, this month we have selected Xtandi and prepared a curated analysis report for our readers
-
Xtandi is indicated for the treatment of certain prostate cancers
-
PharmaShots presents a concise take on the key features of Xtandi with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: Enzalutamide
Dosage Forms & Strengths: Capsules (40mg), Tablets (40mg, 80mg)
Mechanism of Action: Androgen receptor inhibitor
Originator: Astellas
Collaborator: Pfizer
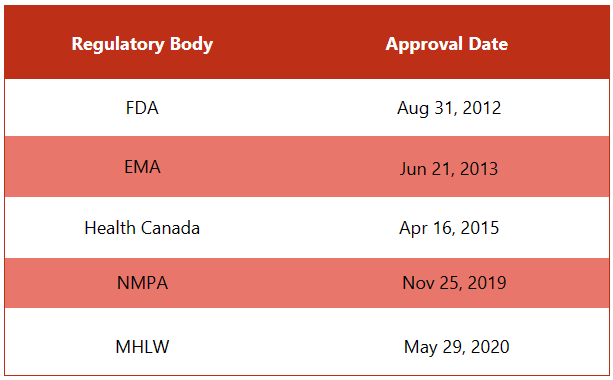
First approvals: The table below depicts the first approvals of Xtandi from different regulatory agencies.
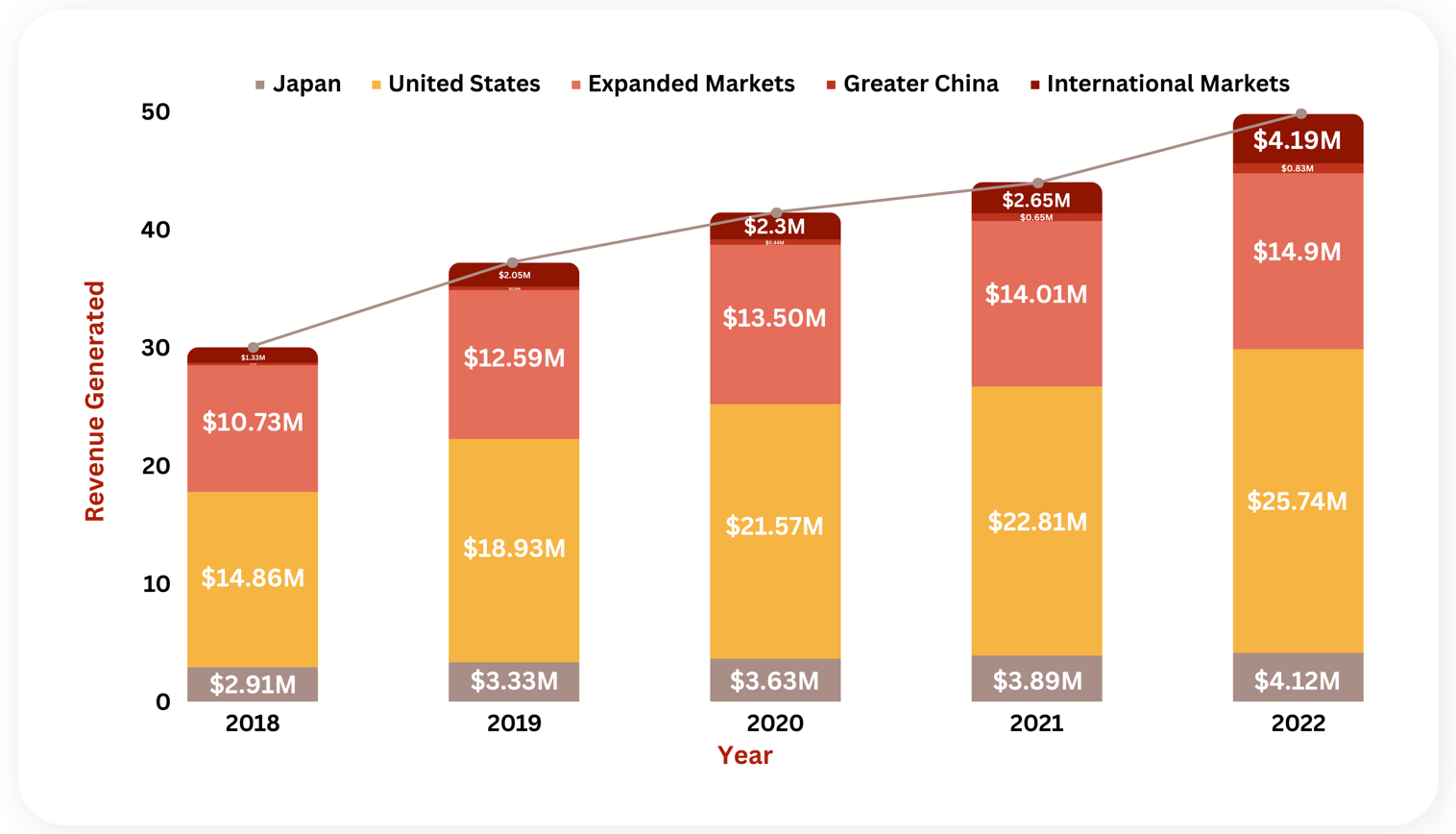
Revenue Analysis
The sales of Astellas’ lead asset, Xtandi have substantially grown to add a huge amount of profit to the company’s overall revenue. In the year 2022, Xtandi generated a total of $49.79M in sales depicting a 13.13% increase from the year 2021. Over 5 years, the highest percentage change in the product’s overall revenue was seen in the year 2019, with a 23.82% increase in the sales as compared to the year 2018. This upsurge in the product’s sales was attributed to the increased use among patients with earlier stages of prostate cancer. Moreover, in the year 2022, the overall sales of Xtandi were contributed by $4.12M from its sales in Japan, $25.74M from the Unites States, $14.9M from the Established Markets, $0.83 from Greater China, and $4.19M from the International Markets.
The following graph illustrates the revenue analysis for the last five year sales of Xtandi.
Approved Indications
Xtandi is a prescription medicine indicated for the treatment of:
-
castration-resistant prostate cancer (US)
-
metastatic castration-sensitive prostate cancer (US)
-
non-metastatic castration-sensitive prostate cancer with BCR at high risk for metastasis (US)
-
Adult men with metastatic hormone-sensitive prostate cancer (mHSPC) combination with ADT (EU)
-
Adult men with high-risk non-metastatic CRPC (EU)
-
Adult men with metastatic CRPC who are asymptomatic or mildly symptomatic after failure of ADT in whom chemotherapy is not yet clinically indicated (EU)
-
Adult men with metastatic CRPC whose disease have progressed on or after docetaxel therapy (EU)
Clinical Trials Analysis
Clinical trial analysis is vital for advancing medical science, improving patient care, and making informed decisions about the safety and efficacy of new treatments. It underpins the foundation of evidence-based medicine and regulatory approval processes, leading to better healthcare outcomes and the development of innovative therapies.
The following dashboard illustrates the clinical trials associated with Xtandi.
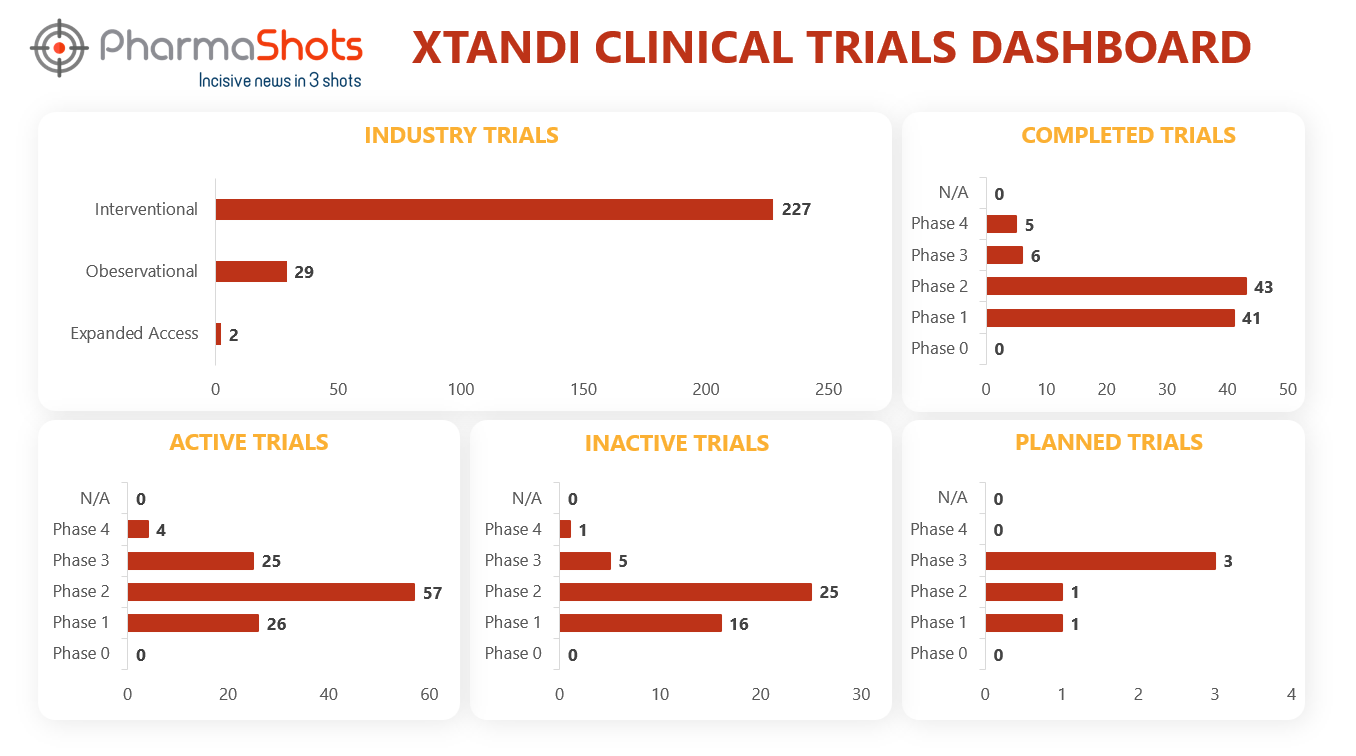
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
Xtandi Trials Representation (Country-wise)
Below graphs depict ongoing trials investigating Xtandi.
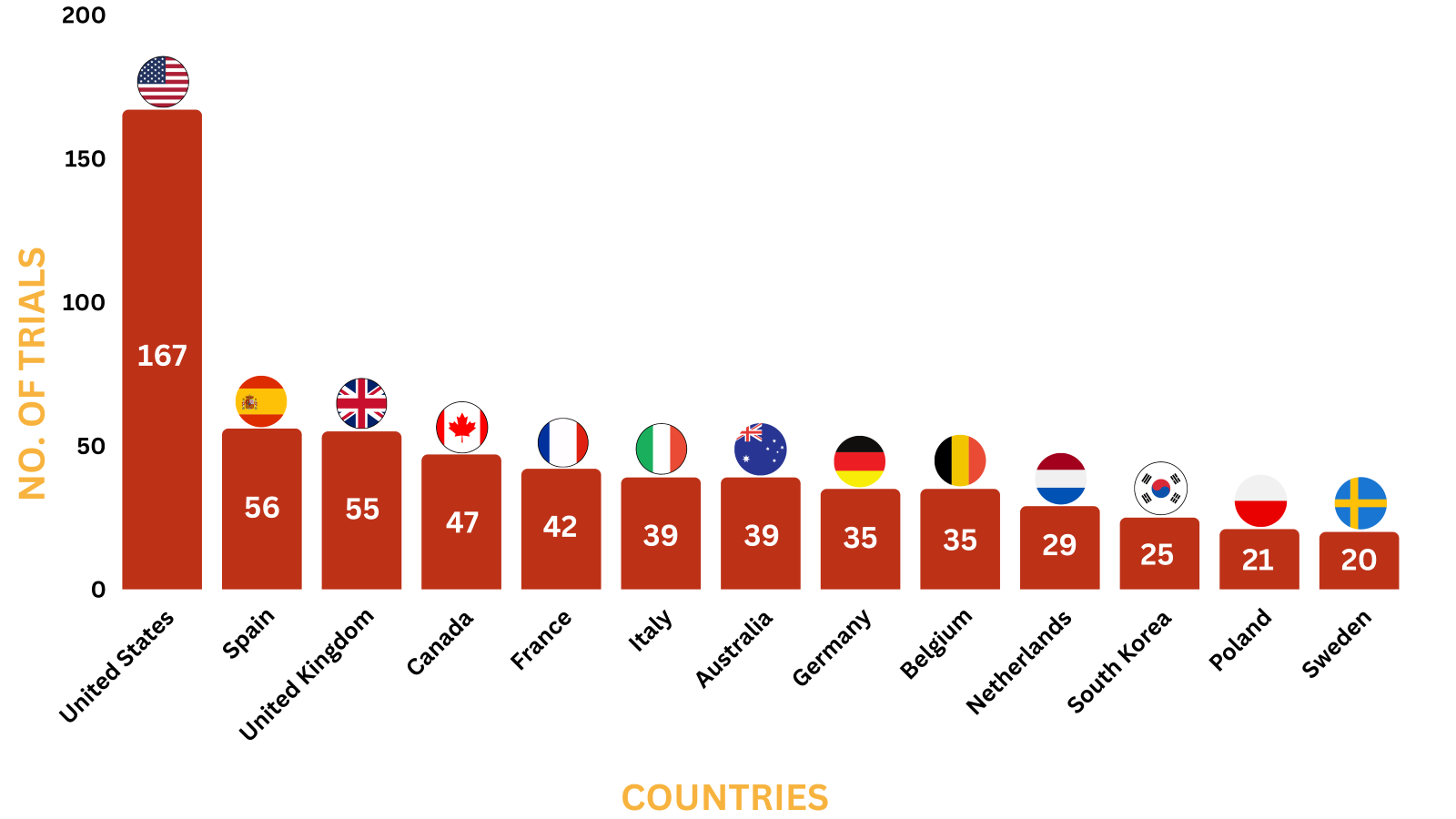
*The data represents trials till Dec 13, 2023
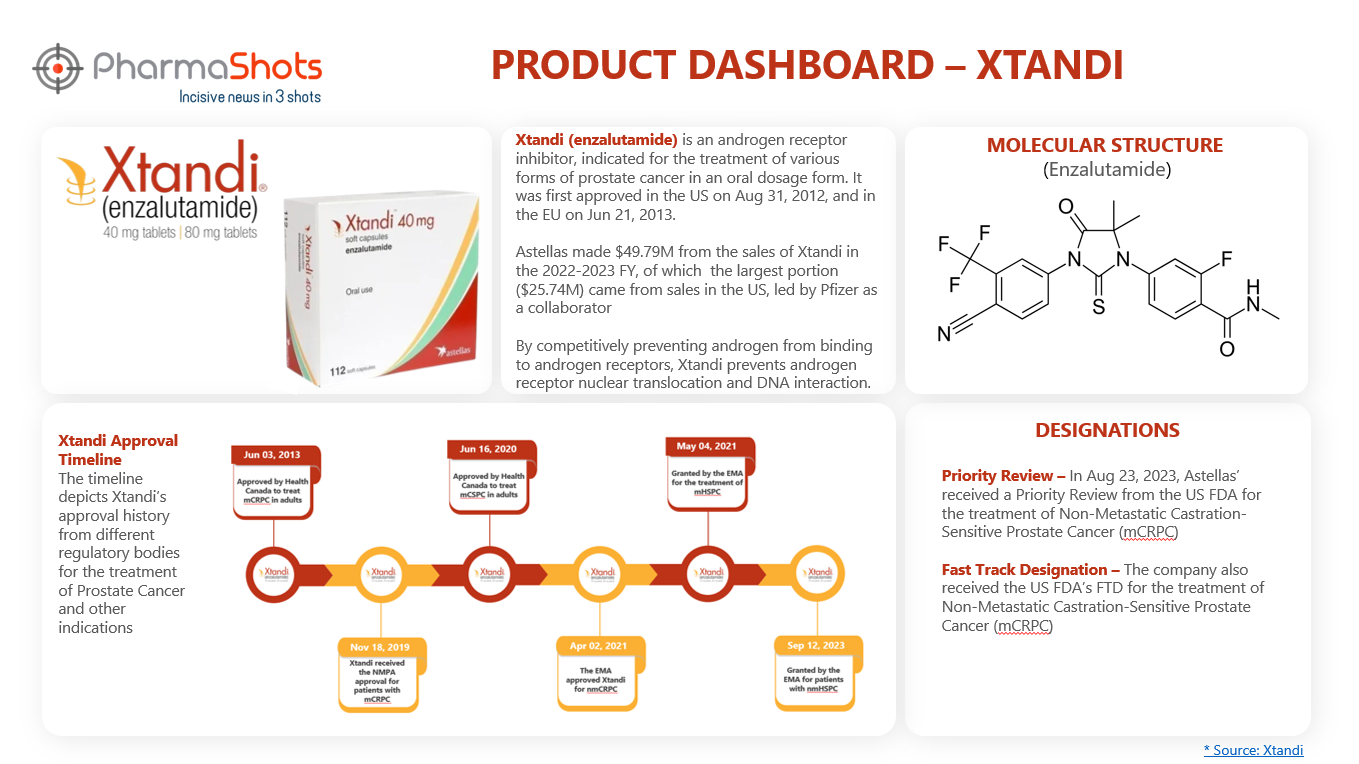
Product Dashboard
PharmaShots presents an illustrative infographic, highlighting essential metrics and pertinent information about Xtandi.
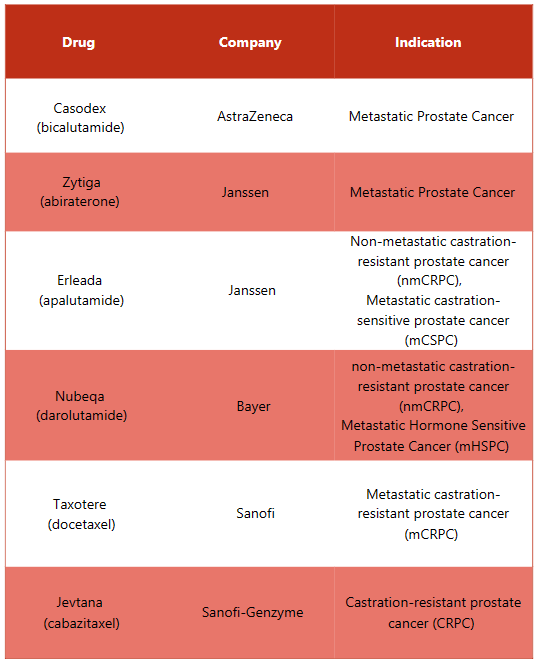
Xtandi Alternative Drugs
In response to Xtandi, several alternative drugs are available in the market to treat different indications. Some of the substitute drugs for Xtandi include:
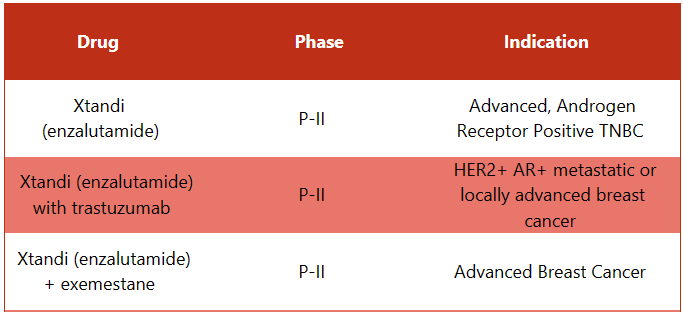
Xtandi Pipeline Analysis
PharmaShots presents an extensive analysis of Xtandi’s pipeline, including the ongoing P-II and P-III studies for various indications. The table below depicts an overview of these studies.
Xtandi SWOT Analysis

Strength:
-
Worldwide Reach: Xtandi is available in one or more approved indications in more than 90 countries, including the US, EU, and Japan. Xtandi’s strong presence in the global market is evident from its annual sales
-
Simple Management: The ease of administration makes Xtandi, the most viable option among the existing medication in patients with prostate cancer and metastatic castration-resistant prostate cancer
-
Effectiveness in treating Prostate Cancer: By demonstrating effectiveness in treating patients with mCRPC, Xtandi ferries staggering support in the patient and KOL community
-
Patent Safeguard: In most of the regions Xtandi’s patent enjoys market exclusivity till 2026 and 2027, providing it a competitive advantage over generics
Weakness:
-
Elevated Expenses: The relatively high cost of medication remains a major weakness for Xtandi, making it inaccessible to many patients around the globe
-
Adverse Drug Events: Like every medication available, Xtandi too displays a few adverse events, which make it difficult for patients with any underlying condition to take the medication
-
Intensely Competitive Treatment Alternatives: Available alternative therapies impact the market for Xtandi with their highly competitive pricing
Opportunities:
-
Research & Development: With advanced research, Xtandi can pave the way to new formulations and combination drugs to advance its market exclusivity and augment its therapeutic potential
-
Rise in Incidence of Prostate Cancer Globally: The rise in incidences of Prostate Cancer worldwide offers a significant advantage to Xtandi over other drugs due to its enhanced global presence and other positive traits
-
Broadened Usage Scenarios: By navigating the usage of Xtandi in other indications, a door to new opportunities can be opened to increase its dominance in the market for the long run
Threats:
-
Innovative Therapeutic Approaches: Emerging medications in prostate cancer and mCRPRC can be a huge challenge to tackle in the future
-
Regulatory Challenges: Gaining acceptance and approval for new formulations and new indications can be challenging for Xtandi
-
Generic Drug Market Competition: With patent expiry, generic drugs would influence the prices and severely impact its market
Patient Stories
Patients' stories are the key resources as they provide a holistic perspective on the impact of medications on individuals' lives, improve healthcare decision-making, and contribute to the overall understanding of the drug's efficacy and safety in real-world settings. We have summarized some of the patients’ stories for Xtandi:
-
WAYNE’S STORY: Wayne was diagnosed with prostate cancer when he was 51 years old. He underwent surgeries and radiation therapies, but still cancer metastasized, that’s when his doctor suggested him to Xtandi. Wayne believes in being a self-advocate for his treatment choices like he was proactive about his treatment and their side effects for his advanced prostate cancer.
-
EVON’S STORY: Evon, a passionate musician, discovered his metastatic prostate cancer at the age of 59 yrs. At the start, he was hesitant to talk about it, but his wife supported him and encouraged him to communicate and discuss the treatment options with his oncologist. They discussed and decided on Xtandi. He talked about Xtandi Doctors’ guide and believes community, support, and having a game plan is important when living with advanced prostate cancer.
-
MAHLON’S STORY: Mahlon, a firefighter, found he had prostate cancer during a routine physical examination. He went for surgical prostate removal, but the cancer came back after 1 year. He followed that with 39 radiation and hormone therapies but after months his PSA started rising again. It is then he researched on his own and discussed Xtandi with his doctor. After careful consideration, they agreed on Xtandi. With some side effects, Mahlon is happy with the results so far as he is starting to find ways to deal with both good and bad days of his life.
KOL* Reviews
KOL reviews offer valuable perspectives on different products and services. These reviews prove beneficial for consumers who engage in product research and prefer to read multiple reviews before making a purchase. Here are a few KOL reviews regarding Xtandi
-
Courtney Bugler, President and CEO of ZERO Prostate Cancer said, "This approval of XTANDI is a promising treatment option for the community, offering a ray of hope to patients and their caregivers during these challenging times."
-
Neal Shore, Director of GenesisCare USA, said, "Previously, treatment options for these BCR patients, especially those who have a high likelihood of developing metastases were limited. The FDA approval of XTANDI for patients with nmCSPC with BCR at high risk of metastasis represents an important advancement whereby an androgen deprivation signaling inhibitor, enzalutamide, has achieved standard of care discussion for patient-physician decision-making."
-
Ahsan Arozullah, Senior Vice President of Astellas said, "With every milestone, our clinical development program has played an instrumental role in changing the course of patients' lives. We are proud that XTANDI can now be offered to a subset of men with nonmetastatic castration-sensitive prostate cancer with biochemical recurrence and at high risk for metastases."
-
Chris Boshoff, Executive Vice President at Pfizer said, "This milestone is a testament to XTANDI's legacy and robust clinical profile, with overall survival demonstrated for patients with metastatic castration-resistant prostate cancer, nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer. With today's approval, we look forward to bringing this therapy to even more patients who have nonmetastatic castration-sensitive prostate cancer at high risk for their cancer metastasizing."
-
Andy Schmeltz, Global President, Pfizer Oncology said, “Today’s approval adds to over a decade of global clinical research aimed at better understanding the potential benefit of XTANDI for men with advanced prostate cancer. The FDA approval marks continued progress to help meet the needs of patients, including men living with metastatic castration-sensitive prostate cancer.”
* Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
- ClinicalTrials.gov
- Drugs.com
- Astellas Newsroom
- PRNewswire
- EMA
- Businesswire
- Medical News Today
- Astellas SEC Filings
Related Post: Top Performing Drug – Cosentyx (November Edition)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














